Aerospace Electronics PCB Assembly & Design Guide
You’ve heard about the many great feats and discoveries in the aerospace industry. You’ve also heard about the tragedies that have happened when a...
3 min read
 Matric Group
:
Nov 08, 2023
Matric Group
:
Nov 08, 2023
 Medical device companies looking for a PCB design and assembly partner have many important factors to consider, all with the end user’s well-being at risk. Finding an experienced, ISO 13485-certified manufacturer is one of the most important.
Medical device companies looking for a PCB design and assembly partner have many important factors to consider, all with the end user’s well-being at risk. Finding an experienced, ISO 13485-certified manufacturer is one of the most important.
In an industry where lives hang in the balance, OEMs like yours need assurance their vendors are capable of meeting the many standards of medical devices. Working with an ISO 13485 contract manufacturer can help OEMs meet the standards of the U.S. Food & Drug Administration’s Quality System Regulation as well as Current Good Manufacturing Practices. But there's more to it than that.
What do ISO standards for medical devices actually entail that makes them so reassuring? What can happen if the certification isn’t there?
You may be asking, if the issue is quality, isn’t ISO 9001 -- and its emphasis on continuous improvement and customer satisfaction -- enough? Not in the medical world.
If a contractor has ISO 13485 certification, it also has ISO 9001 certification. However, ISO 13485 adds critical requirements around devices, documentation, and safety.
A standard for quality management systems aimed specifically at medical devices, ISO 13485:2016 (the full name of the latest version) focuses covering your butt in case of an issue. More specifically, the emphasis is on managing risk -- on foreseeing and fixing problems before they occur:
In other words, ISO 13485 represents an exhaustive effort to make sure each component meets rigorous medical device manufacturing standards.
In many cases, the electronics contract manufacturer is ISO 13485-certified, but the OEM ordering that PCB (printed circuit board) assembly isn't. OEMs are strictly regulated by FDA medical device regulatory requirements and have to comply with Current Good Manufacturing Practices (CGMP), which focuses more on the finished product than the guts within.
The spirit of those same guidelines are baked into ISO 13485, so certified manufacturers have shown to auditors that they’ve applied CGMP, too. That’s yet another valuable layer of support behind a medical device manufacturer!
 ISO 13485 Certification: a Critical Extra Layer
ISO 13485 Certification: a Critical Extra LayerIPC (another standards org you're probably familiar with) lists medical equipment as Class 3 electronics. Unsurprisingly, this is because failure of a circuit board or component could put lives at risk.
A manufacturer doesn’t have to be an ISO 13485-certified company to meet medical device manufacturing regulations. And it doesn’t have to be certified to make and sell medical device components or the finished devices.
However, a medical device manufacturing certification tells everyone -- regulators and customers included -- that your electronics underwent an objective evaluation by a third party and are proven to be safe.
An electronics manufacturer can be part of a long supply chain. With a specific ISO certification for medical devices on its side, a contractor can assure its end of the deal meets the highest standards.
Such rigor is not mandatory -- you can legally release a product to the market without using a certified supplier. It’s up to you whether to add this layer of quality control.
So, what could go wrong if this “unnecessary” certification isn’t accomplished? What if a component manufacturer doesn’t have ISO 13485 certification and something goes amiss when the device is used in the emergency room?
Chances are the FDA will investigate the OEM. And that means the OEM will need lots of records to verify compliance with regulated processes. If the medical device contract manufacturer can say with confidence that all the necessary records are available, any OEM will sleep easier.
OEMs want validation of a contractor’s manufacturing processes as a way to help validate their own final products. A PCB manufacturer’s records must meet strict medical requirements.
Some contractors go a step farther in the validation process. They audit their critical component suppliers annually to make sure they meet the contractor’s testing and inspection standards. Working with such a contract manufacturer is a great way to reinforce the safety chain without lifting a finger.
Because of strict industry requirements, there are already many challenges facing medical device manufacturers. Why add another by cutting corners?
Companies responsible for selling finished medical devices score some important benefits when using a ISO 13485 certified manufacturer:
Thorough, clear understanding among all parties of the device’s end use, avoiding errors in design and manufacturing
Risk analysis methods that are well-defined and effective to avoid expensive safety problems that appear after final production
Focus on the end-user to ensure ease of use, safety, and effectiveness
Audits to make contractors fully accountable for product quality and safety
There are many benefits to any OEM seeking the services of a turnkey PCB manufacturer. But if you're in the medical industry, first question #1 when courting a medical device component manufacturer should always be: “Are you ISO 13485 certified?” The answer carries a lot of weight.
(Editor's note: This article was originally published in March 2019 and was updated in November 2023 to reflect current information.)

You’ve heard about the many great feats and discoveries in the aerospace industry. You’ve also heard about the tragedies that have happened when a...

“How will my idea go from conception to finished product at your company?” It’s a question we hear a lot, and a good one. It’s natural to want to...
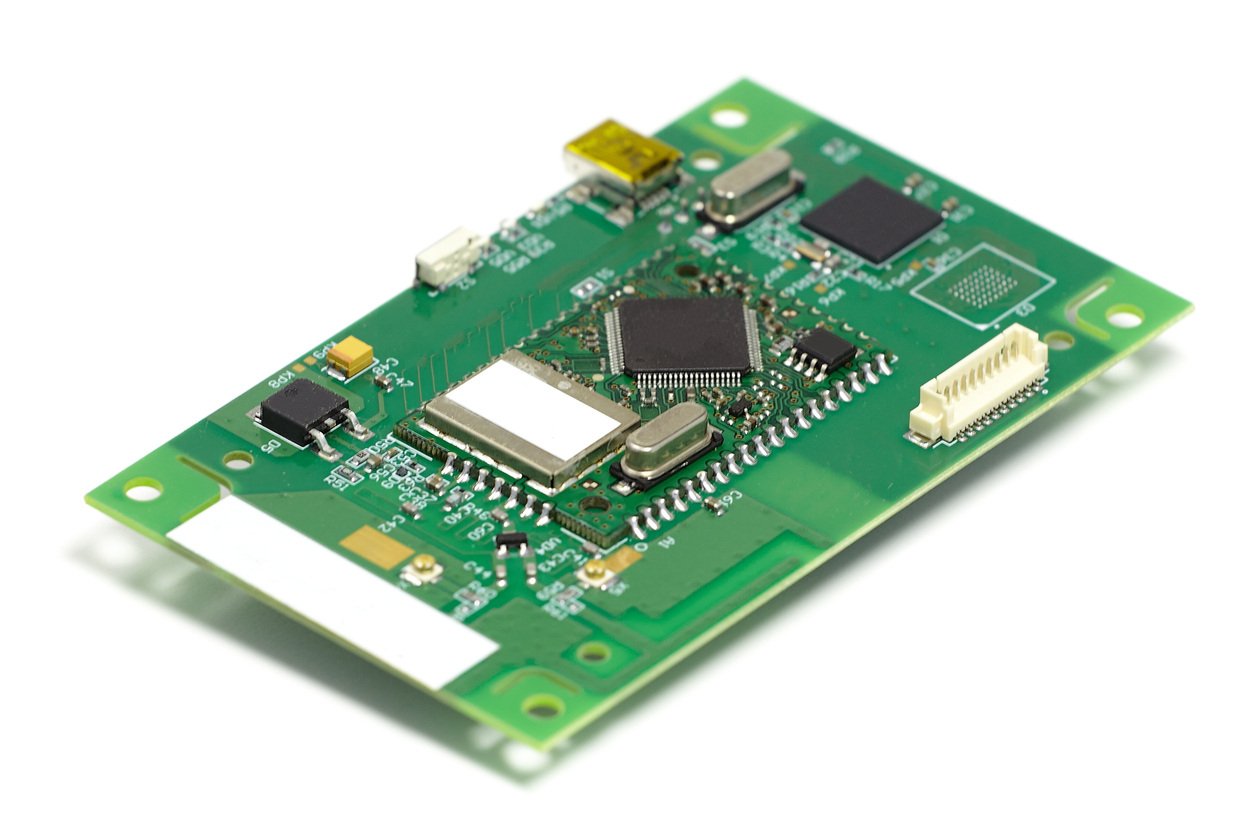
Bringing a new electronic product to market is no small feat. From intricate circuit board designs to complex cable assemblies, every detail must...